SafetyGraft® Autologous Bone Flap Storage
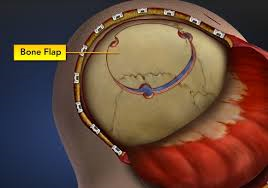
California Transplant Services, Inc. provides the SafetyGraft® Autologous Tissue Storage System for skull flaps recovered from trauma and surgical patients. Head injury and neurosurgical surgical patients suffering from brain swelling may have to have a portion of the skull bone stored for re-implant at a later date. We provide ultra low temperature storage at below -70C in monitored calibrated ULT freezers in our FDA registered and state licensed tissue bank laboratory.
This service should be set up in advance of the recovery of autologous tissue to enable training of the operating room staff of the required procedure to be followed.
Set up includes comprehensive staff training in preservation technique, record keeping, labeling, shipment packout and a written hospital storage agreement.
We have provided services since 1994 to level 1 & level 2 trauma centers in California, Texas, Washington, Arizona and Nevada in addition to secondary facilities throughout western United States.
SafetyGraft® has a nationwide network of couriers who coordinate with the airlines and FedEx to expedite your shipment, usually within a few hours to our facility. SafetyGraft® is a TSA qualified “Known Shipper” with the airlines to enable rapid deployment of all shipments.
Key Benefits
- No risk of tissue rejection, since it is SafetyGraft® stored autologous tissue.
- Reduced surgical implant time since bone plate fits exactly to the patient, and does not need to be fashioned out of donor bone or synthetic materials.
- SafetyGraft® storage facility is responsible for the management of storage and delivery to surgical facility at the time of implant regardless of the implant location, worldwide.
- SafetyGraft® Storage and preservation kits are provided to the procuring hospital at no charge. Charges are only incurred at the time of recovery of tissue for storage.
- No chance of secondary wound site infection from storing bone in the abdomen.
- We provide SafetyGraft® training and educational aids for operating room staff.
Additional information on FDA registration requirements.
To obtain more information please call us at 800-928-4778